Our competences
Medical technology “Made in Germany”
Over the years, in cooperation with many customers, development and design partners, we have developed countless devices that have always belonged to the respective top class. In the process, we have gained experience with enclosures of all technologies: Sheet metal, continuous casting, deep-drawn parts, injection moulding, … This enables us to select the optimal enclosure technology, adapted to the respective framework conditions.
Another focus is ergonomics, which always plays a central role in our enclosure developments. In recent years, touch technology was used at gbo at a very early stage, but only in combination with intelligent operating concepts that avoid “rummaging” through endless menu structures.
 It is almost a matter of course that we operate our own in-house hardware and software development. Special care is taken to ensure that only components, modules and parts are used that guarantee long-term availability in the rapidly changing environment.
It is almost a matter of course that we operate our own in-house hardware and software development. Special care is taken to ensure that only components, modules and parts are used that guarantee long-term availability in the rapidly changing environment.
To be a competent and complete manufacturer of physical therapy devices, one should be proficient in other core technologies besides device construction.
gbo is fully positioned in ultrasound technology. We work together with specialists in piezoelectric ceramics and develop our ultrasonic heads based on this. This includes the vibration and radiation-optimised design of the resonators, the control electronics, the software algorithms and the housing design.
The necessary know-how and the appropriate measuring equipment are available to verify the quality of the resulting product. In manufacturing, the process steps of joining and generating the required vibration quality are among the critical steps.
In addition to ultrasound, high-frequency technology is part of the gbo technologyportfolio. Short-wave devices (27 MHz), microwave devices (2.45 GHz) and their respective applicators are developed here.
Of course, all aspects of electrotherapy are at the centre of our know-how. This concerns the generation of all forms of therapy such as low frequency, medium frequency, interference and gbo’s ownpatented high tone therapy. Not insignificant are also the suitable applicators with partic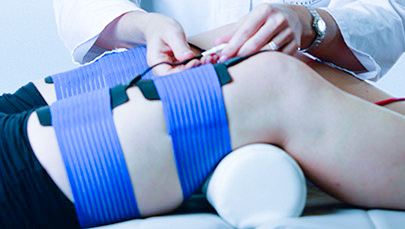 ularly low-resistance biocompatible silicones and suction applicators, both in classical form and according to the Venturi principle.
ularly low-resistance biocompatible silicones and suction applicators, both in classical form and according to the Venturi principle.
gbo Medizintechnik AG has a documented quality management system and is certified according to DIN EN ISO 13485:2012 by TÜV Süd. And this throughout, from development to production and distribution. We also have relevant experience with approvals abroad, such as Japan, USA, Russia and many more.On this basis, we can document the products we develop in conformity withstandards and “approve” them via corresponding declarations of conformity. Of course, our OEM customers also benefit from this.


